Précisez une action ou une cryptomonnaie dans la barre de recherche pour obtenir un récapitulatif

Verrica Pharmaceuticals Inc
1NEVerrica Pharmaceuticals Inc., a clinical-stage dermatology therapeutics company, develops medications for the treatment of skin diseases in the United States. Its product pipeline comprises YCANTH (VP-102), which is in phase III clinical trial for the treatment of common warts; and has completed phase II clinical trial for the treatment of external genital warts. The company also develops VP-315, an oncolytic peptide-based injectable therapy, which is in phase II clinical trial for the treatment of dermatology oncologic conditions which includes basal cell carcinoma; and VP-103, a cantharidin-based product candidate for the treatment of plantar warts and is in phase II clinical trial. In addition, it offers YCANTH for the treatment of molluscum contagiosum. The company has a collaboration and license agreement with Torii Pharmaceutical Co., Ltd. for the development and commercialization of its product candidates for the treatment of molluscum contagiosum and common warts in Japan, including VP-102; and a license agreement with Lytix Biopharma AS to develop and commercialize VP-315 for dermatological oncology indications, such as non-metastatic melanoma and non-metastatic merkel cell carcinoma. Verrica Pharmaceuticals Inc. was incorporated in 2013 and is headquartered in West Chester, Pennsylvania. Address: 44 West Gay Street, West Chester, PA, United States, 19380
Analytics
Objectif de Cours de WallStreet
9.25 USDRatio C/B
–Rendement du dividende
–Année actuelle
L'année dernière
Trimestre en cours
Le dernier trimestre
Année actuelle
L'année dernière
Trimestre en cours
Le dernier trimestre
Chiffres clés 1NE
Analyse des dividendes 1NE
Croissance des dividendes sur 5 ans
–Croissance continue
–Ratio de distribution Moyenne sur 5 ans
–Historique des dividendes 1NE
Valorisation des titres 1NE
financières 1NE
| Résultats | 2019 | Dynamique |




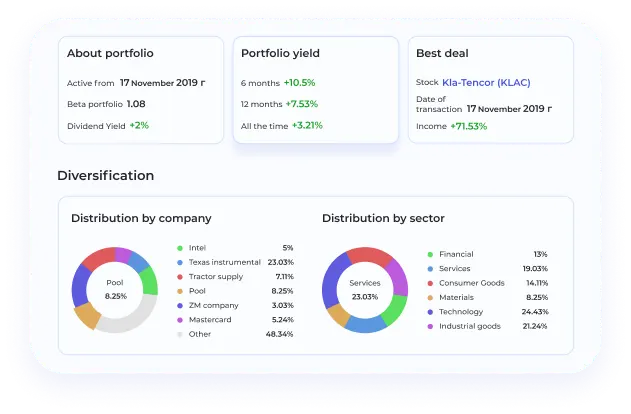




 BeatStart
BeatStart