GNI Group Ltd
2160GNI Group Ltd. engages in the research, development, manufacture, and sale of pharmaceutical drugs in Japan and internationally. It provides Etuary for the treatment of idiopathic pulmonary fibrosis. The company is also developing Etuary, which is in Phase III clinical trial for the treatment of connective tissue associated interstitial lung disease; and in clinical trial pre-phase III clinical trial pilot study for the treatment of radiation pneumonitis, as well as for the treatment of diabetic nephropathy. In addition, it is involved in developing F351, which is in Phase III clinical trial for the treatment of liver fibrosis; and F573 that is in phase II for the treatment of acute on chronic liver failure, as well as Tamibarotene for the treatment of acute promyelocytic leukemia. GNI Group Ltd. was incorporated in 2001 and is headquartered in Tokyo, Japan. Address: Nihonbashi-Honcho YS Building, Tokyo, Japan, 103-0023




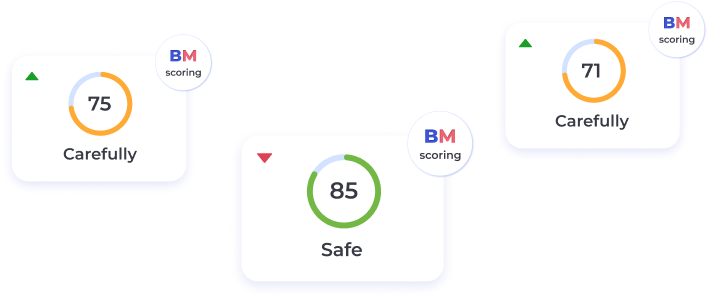

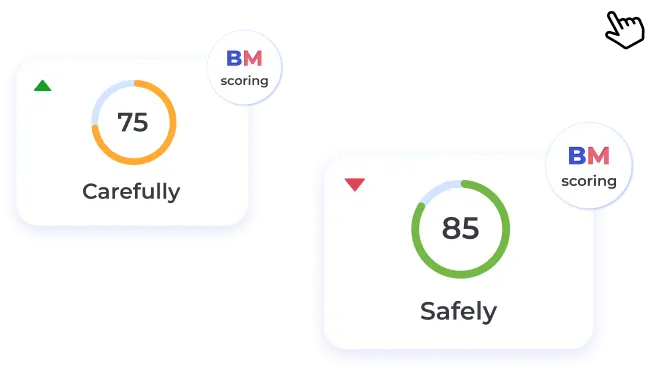
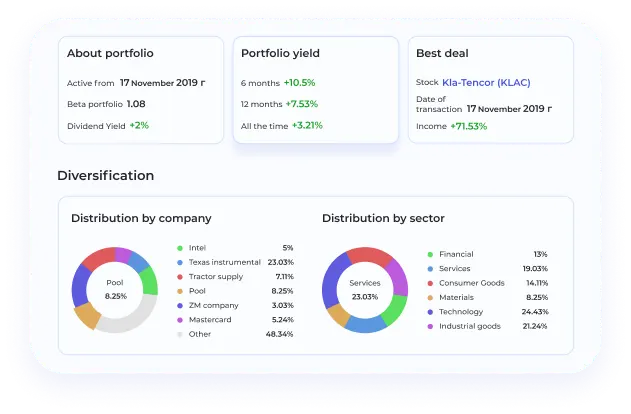




 BeatStart
BeatStart