Regenxbio Inc
RB0REGENXBIO Inc., a clinical-stage biotechnology company, provides gene therapies that deliver functional genes to cells with genetic defects in the United States. Its gene therapy product candidates are based on NAV Technology Platform, a proprietary adeno-associated virus gene delivery platform. The company's products in pipeline includes ABBV-RGX-314 for the treatment of wet age-related macular degeneration, diabetic retinopathy, and other chronic retinal diseases; and RGX-202, which is in Phase I/II clinical trial for the treatment of Duchenne muscular dystrophy. It also develops RGX-121 for the treatment of mucopolysaccharidosis type II that is in Phase III clinical trial; RGX-111 for treating mucopolysaccharidosis type I; RGX-181 for the treatment of late infantile neuronal ceroid lipofuscinosis type II; and RGX-381 to treat the ocular manifestations of CLN2 disease. In addition, the company licenses its NAV Technology Platform to other biotechnology and pharmaceutical companies. Further, it has a collaboration and license agreement with AbbVie Global Enterprises Ltd. to develop ABBV-RGX-314 outside the United States. REGENXBIO Inc. was incorporated in 2008 and is headquartered in Rockville, Maryland. Address: 9804 Medical Center Drive, Rockville, MD, United States, 20850




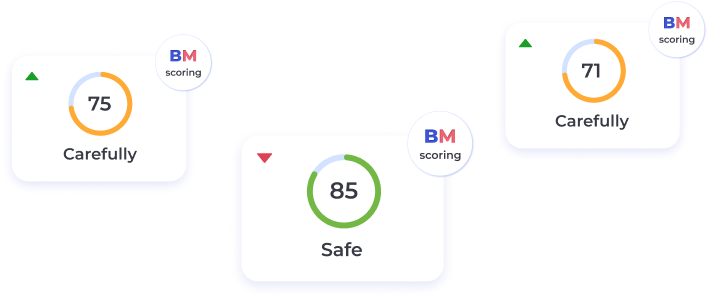

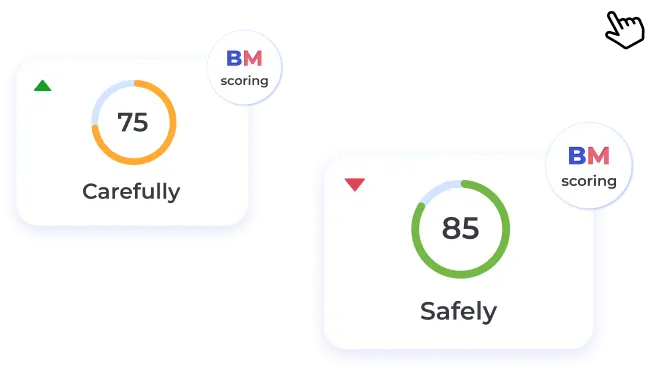
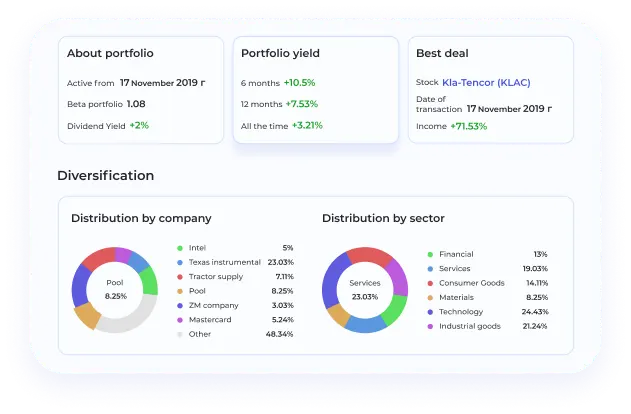




 BeatStart
BeatStart