Ocugen Inc
OCGNOcugen, Inc., a clinical-stage biopharmaceutical company, focuses on discovering, developing, and commercializing novel gene and cell therapies and vaccines that improve patients' health. The company's pipeline product includes OCU400, a novel gene therapy product candidate restoring retinal integrity and function across a range of genetically diverse inherited retinal diseases, currently under Phase 3 trials for the treatment of retinitis pigmentosa and Phase 1/2 trials for the treatment of leber congenital amaurosis; OCU410, a gene therapy under phase 1/2 for the treatment of dry age-related macular degeneration (AMD); and OCU410ST, a gene therapy under phase 1/2 for the treatment of Stargardt disease. It is also involved in the development of OCU200, a novel fusion protein that is in preclinical development stage for the treatment of diabetic macular edema, diabetic retinopathy, and wet age-related macular degeneration; and NeoCart, an autologous chondrocyte-derived neocartilage, currently under Phase 3 studies indicated for the repair of knee cartilage injuries in adult. In addition, the company is developing OCU500, a COVID-19 vaccine; OCU510, a seasonal quadrivalent flu vaccine; and OCU520, a combination quadrivalent seasonal flu and COVID-19 vaccine. It has collaboration agreements with National Institute of Allergy and Infectious Diseases for early clinical studies for the OCU500 program; and a strategic partnership with CanSino Biologics Inc. for manufacturing its modifier gene therapy pipeline product candidates. The company was founded in 2013 and is headquartered in Malvern, Pennsylvania. Address: 11 Great Valley Parkway, Malvern, PA, United States, 19355





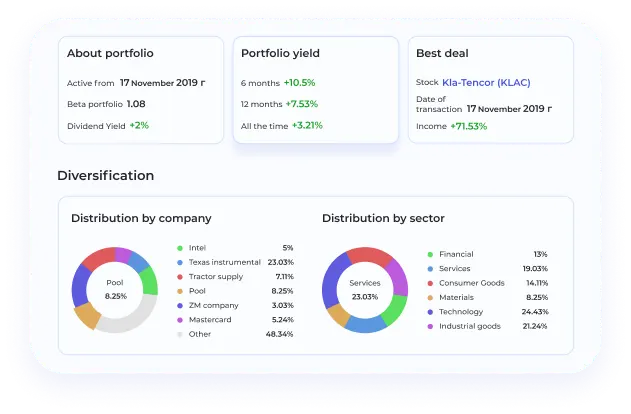




 BeatStart
BeatStart