Kirjoita osake tai kryptovaluutta hakupalkkiin saadaksesi yhteenvedon

Cara Therapeutic
69CCara Therapeutics, Inc., a development-stage biopharmaceutical company, focuses on developing and commercializing therapeutics treatment of chronic pruritus in the United States. The company's lead product is KORSUVA (difelikefalin) injection for the treatment of moderate-to-severe pruritus associated with chronic kidney disease (CKD) in adults undergoing hemodialysis. It also develops Oral difelikefalin, which is in Phase II/III clinical trial to treat chronic pruritus with notalgia paresthetica. The company has license agreements with Maruishi Pharmaceutical Co., Ltd to develop, manufacture, and commercialize drug products containing difelikefalin for acute pain and uremic pruritus in Japan; Vifor Fresenius Medical Care Renal Pharma Ltd. development and commercialization of KORSUVA injection for the treatment of moderate-to-severe pruritus in adult patients undergoing hemodialysis; and Chong Kun Dang Pharmaceutical Corporation to develop, manufacture, and commercialize drug products containing difelikefalin in South Korea. Cara Therapeutics, Inc. was incorporated in 2004 and is based in Stamford, Connecticut. Address: 400 Atlantic Street, Stamford, CT, United States, 06901
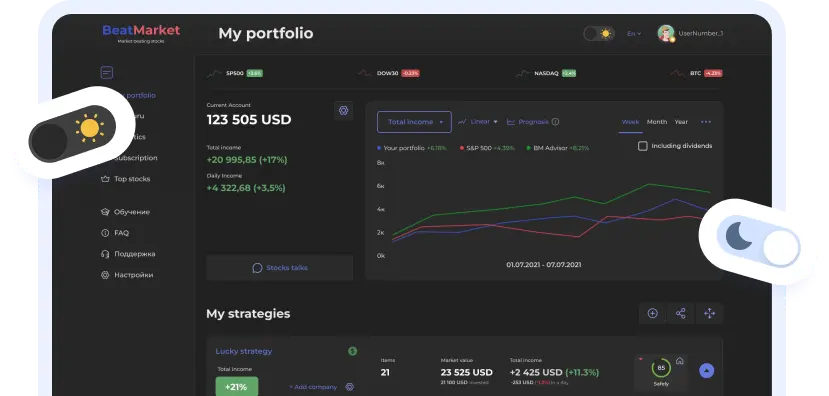
Analytics
WallStreetin tavoitehinta
1.01 USDP/E-suhde
–Osinkotuotto
–Kuluva vuosi
Edellinen vuosi
Nykyinen vuosineljännes
Viimeinen vuosineljännes
Kuluva vuosi
Edellinen vuosi
Nykyinen vuosineljännes
Viimeinen vuosineljännes
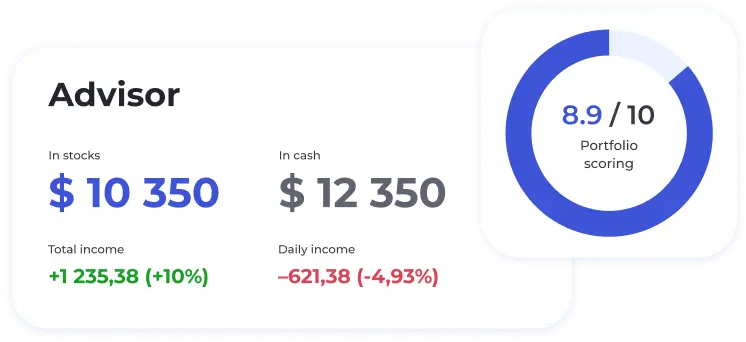
Avainluvut 69C
Osinkoanalytiikka 69C
Osinkojen kasvu 5 vuoden aikana
–Jatkuva kasvu
–Maksusuhde 5 vuoden keskiarvo
–Osinkohistoria 69C
Osakkeen arvostus 69C
Talousasiat 69C
| Tuloksia | 2019 | Dynamiikka |



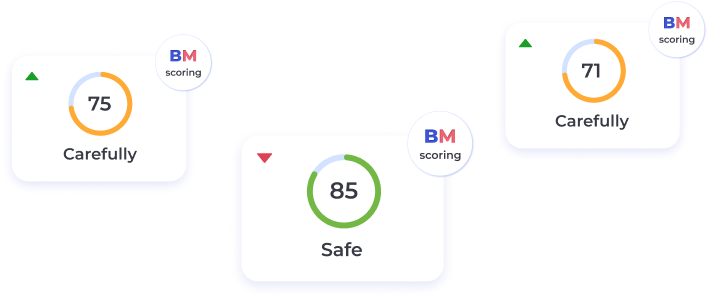

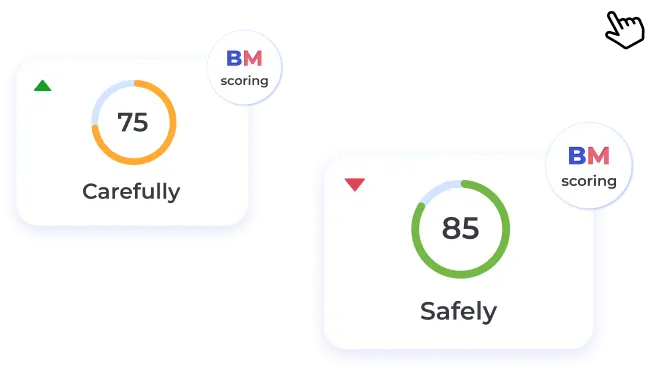
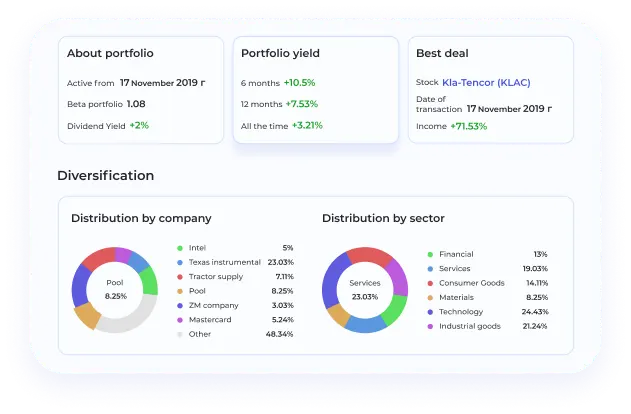




 BeatStart
BeatStart